Development of Replacement Materials for Damaged and Diseased Blood Vessels
Wayne Carver, Professor and Chair, Department Cell Biology and Anatomy, University of South Carolina - PI
A Stimulus Research Program Award Supported by the SC EPSCoR Program
(SC EPSCoR SRP Award 18-SR01)
The Research Team:
- Wayne Carver, Professor and Chair, Department Cell Biology and Anatomy, University of South Carolina
- John Eberth, Associate Professor, Department of Cell Biology and Anatomy, University of South Carolina
- Derrick Swinton, Professor and Interim Dean, Department of Chemistry, Claflin University
- Matthew Stern, Associate Professor, Department of Biology, Winthrop University
- William Richardson, Assistant Professor, Department of Bioengineering, Clemson University
Worldwide over 600,000 damaged or diseased blood vessels are surgically replaced annually. For larger blood vessels like the aorta, synthetic materials such as Dacron have a high degree of success for surgical repair of the damaged vessel. Replacement of smaller blood vessels such as the coronary arteries with synthetic materials often leads to failure due in part to occlusion of the blood vessel. Better materials are direly needed for replacement or repair of small diameter blood vessels in humans. Over the past several years, decellularization of existing tissues or organs has become a popular approach to produce scaffolds that can be used for tissue engineering and regenerative medicine. Such scaffolds can be recellularized with stem cells prior to transplantation into patients or used directly without cells. This approach has several advantages including the fact that decellularization largely retains the biomechanical properties of the tissue and, if the endogenous cells are effectively removed, reduces the immune response to the scaffold. A wide array of procedures have been used to decellularize tissues including the use of chemicals such as detergents, nucleases and proteases. To date, the use of decellularization to generate replacement materials for small blood vessels has received little attention and procedures for decellularization of these are far from standardized.
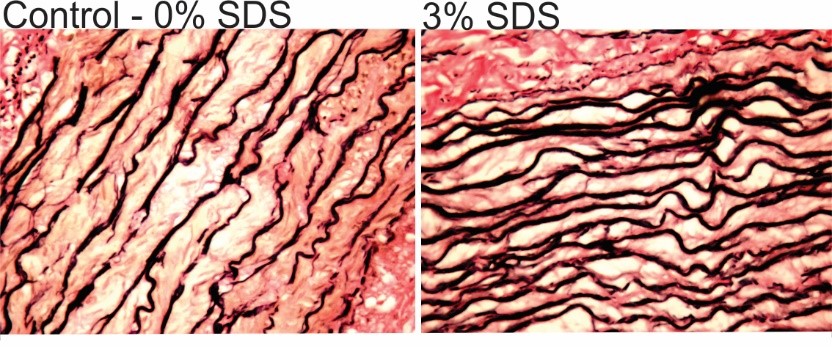
Optimization of procedures to effectively decellularize animal blood vessels for use as replacement materials in humans involves the careful analysis of the effects of decellularization parameters on the properties of the resulting scaffold. A team of researchers with diverse backgrounds in chemistry, vascular cell biology and engineering led by Dr. Wayne Carver of the University of South Carolina School of Medicine has been addressing this problem with funding through the South Carolina EPSCoR Stimulus program. This team has utilized porcine (pig) internal thoracic arteries, which have similar structural and biomechanical properties to human coronary arteries as a starting material for decellularization with the ultimate goal of developing a product that can be readily used for vascular replacement surgery. Studies have been carried out to assess the effects of varying concentrations of anionic detergent (sodium dodecyl sulfate or SDS) and other parameters on the effectiveness of decellularization and on the structural, biomechanical and biochemical properties of the resulting scaffolds. Dr. Carver’s lab has demonstrated that incubation in relatively high concentrations of detergent are required to thoroughly decellularize the porcine internal thoracic arteries. Treatment with high concentrations of detergent does not appear to remove extracellular fibers from the tissue as indicated by histological staining of elastic fibers in tissue sections; however, the fibers appear more densely organized due to removal of cells from the tissue.
 |
|
Figure 1. Histological staining of tissue sections from control and detergent-treated blood vessels illustrating intact elastic fibers following treatment (dark staining structures). |
Dr. Matthew Stern from the Department of Biology at Winthrop University has an extensive background in tissue engineering including the production of natural and synthetic scaffolds and incorporation of stem cells into these. Dr. Stern’s lab is assisting in the structural characterization of scaffolds produced by decellularization via analysis with scanning electron microscopy. Importantly, decellularization with high concentrations of detergent is associated with generation of large pores (Figure 2) within the scaffold that may be advantageous during infiltration of new cells into the scaffold (recellularization), but may also alter its biomechanical properties. To simulate recellularization of the scaffolds by the patient’s cells, Dr. Stern’s lab is also evaluating the ability of vascular cells to adhere to and migrate within the engineered scaffolds. These studies have demonstrated that the high concentrations of detergents that provide most effective decellularization of the porcine tissue are toxic to the cells during recellularization.
 |
|
Figure 2. Scanning electron microscopic image of a detergent-treated blood vessel illustrating abundant collagen fibers. Note large spaces created between the fibers by detergent treatment. |
Advancing decellularized scaffolds as replacement materials for human arteries requires a thorough understanding of vascular biomechanics and remodeling, the expertise of Dr. John Eberth an Associate Professor at the University of South Carolina School of Medicine. It is clear from the research of Dr. Eberth and others that matching the biomechanical properties of the engineered scaffold to those of the normal blood vessel greatly enhances the likelihood of surgical success. Dr. Eberth’s lab has demonstrated that low concentrations of detergent have little effect on the biomechanical properties of scaffolds; however, these properties are altered with higher detergent concentrations. It is well known that the mechanical properties of biological materials is largely dependent on the composition and organization of the extracellular matrix. Dr. Derrick Swinton, Chair of the Department of Chemistry at Claflin University is applying his expertise in proteomics to analyze the protein composition of decellularized scaffolds. Specifically this approach addresses the effects of the decellularization process on retention and degradation of the extracellular matrix proteins of the scaffold. Initial studies have demonstrated substantial differences between decellularized scaffolds and control blood vessels with much fewer differences across the concentrations of detergent used in these studies. This project formed part of the foundation for a successful instrumentation grant to the Department of Defense to obtain a High Resolution Mass Spectrometry system at Claflin University.
While high concentrations of detergent provide the most effective removal of cells from the porcine tissues, these also appear to remove or degrade components of the extracellular matrix critical to scaffold integrity. Residual detergent in the scaffolds also are toxic to cells during the recellularization process. Optimization of decellularization procedures is greatly accelerated by the ability to predict the effects of specific decellularization parameters on desired outcomes. Dr. William Richardson from the Department of Bioengineering at Clemson University has extensive experience in computational modeling and simulation of biological processes. His analysis of quantitative outcomes related to scaffold structural, mechanical and biochemical properties allows the investigative team to adjust specific parameters including detergent concentration to generate scaffolds with desired properties.
Clearly, development of methods that effectively decellularize tissues in the absence of detergents or with low levels of detergent would greatly impact this field. The stimulus protect team has initiated a collaboration with Dr. Michael Matthews in the Department of Chemical Engineering at the University of South Carolina. Dr. Matthews is a renowned researcher in the field of supercritical carbon dioxide, which has been shown to be effective at decontamination of biological materials and in limited studies has been suggested to enhance the decellularization of tissues. Initial studies indicate that treatment of porcine internal thoracic arteries with supercritical carbon dioxide as part of the decellularization process promotes effective decellularization with much lower detergent concentrations. Ongoing studies are underway to integrate supercritical carbon dioxide treatment into an effective decellularization process.
These studies address an important clinical problem as cardiovascular disease remains the leading cause of death worldwide. Development of materials that could be readily used for repair of damaged or diseased blood vessels would have a substantial impact in reducing deaths due to cardiovascular disease.
August 2020
