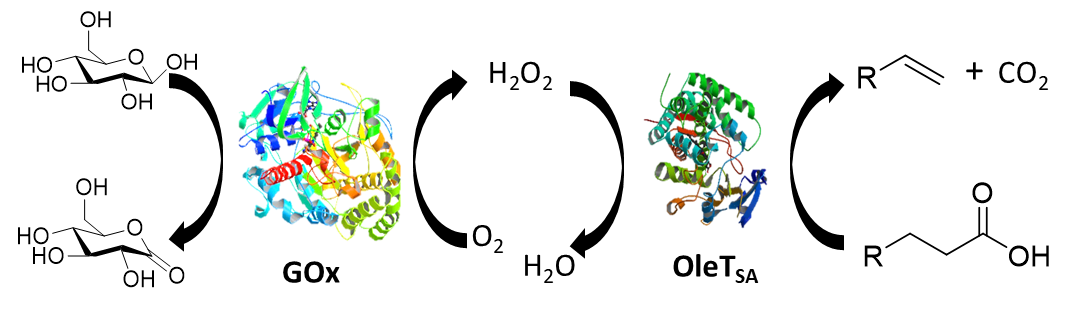Research Focus On Dr. Qian Wang
Carolina Distinguished Professor
Department of Chemistry and Biochemistry
University of South Carolina
Multiscale controlled assembly of functional proteins with polymers for biomaterials development
Dr. Qian Wang and his Research Team are working on the controlled assembly of functional proteins with synthetic polymers. In this work, polymer–protein core–shell nanoparticles have been explored for enzyme immobilization.
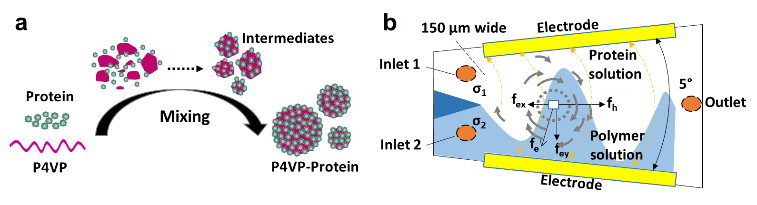
Functional polymer-protein nanoparticles (NPs) have broad applications in biotechnology and nanotechnology. The interaction between functional protein with synthetic molecules guides the assembling process (Figure 1a). Working with Professor Guiren Wang (USC), we have developed an electrokinetics (EK) based microfluidic reactor with fast mixing is explored to assemble functional proteins with polymers in an ethanol/water co-solvent system (Figure 1b). The resultant NPs show significantly improved size distribution by comparison with the ones prepared using conventional bulk method, while the NPs size can be tuned by adjusting the mass ratio of polymer to protein. The functionalities of the assembled proteins are sustained upon the EK based microfluidic mixing, indicating the application potential of our method in the controlled assembly of different functional proteins.
To demonstrate the application potential of the protein assemblies, we focused our study in the synthesis of biofuels using a prototypical enzyme, cytochrome P450 OleT, a fatty acid decarboxylase, which is an industrially important enzyme for biofuel and synthetic applications. This study was in collaboration with Professor Thomas Makris at USC. An artificial enzyme cascade composed of glucose oxidase (GOx) and OleTSA from Staphylococcus aureus was developed for efficient terminal alkene production (Figure 2). The co-assembly of the GOx/OleTSA enzymes with a pyridine-grafted polymer, forming polymer-dual enzymes nanoparticles, displays improved activity compared to the free enzyme. This two-fold strategy provides a simple and efficient system to transform a naturally abundant feedstock to industrially important chemicals.
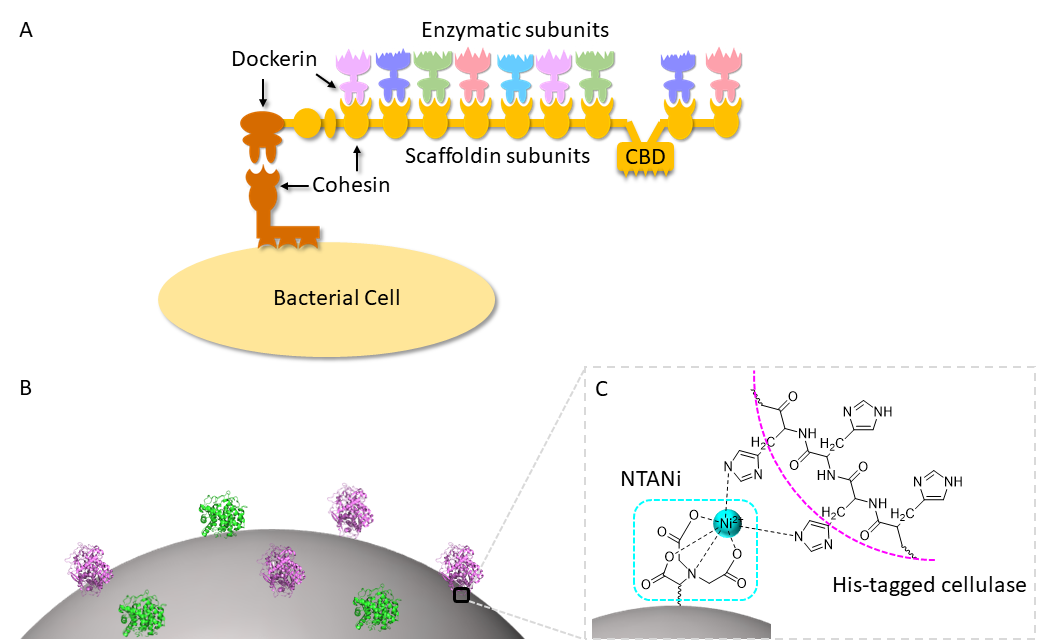
In addition, we have developed functional polymeric micelles for immobilizing His6-tagged cellulases with controlled spatial orientation of enzymes, resulting in “artificial cellulosomes” for effective cellulose hydrolysis (Figure 3A). Poly(styrene)-b-poly(styrene-alt-maleic anhydride) was prepared through one-pot reversible addition–fragmentation chain-transfer polymerization and modified with nitrilotriacetic acid (NTA) to afford an amphiphilic block copolymer. The self-assembled polymer could successfully capture His6-tagged cellulases and form hierarchically structured core–shell nanoparticles with cellulases as the corona (Figure 3B,C). Because the anchored enzymes are site-specifically oriented and in close proximity, synergistic catalysis that results in over twofold activity enhancement has been achieved.
The significance of the project and plans for future work: This is collaborative effort between experimentalists and theoreticians to investigate the nano-scale assembly of functional proteins and synthetic materials. Experimentalists are working on the genetic and chemical modification of proteins and the controlled assembly. Theoreticians are using DFT to create a theoretical model of the biopolymer-synthetic molecule interaction, which can help us to understand the assembly process. To better understand above process, we will investigate the general phenomena of synthetic molecules interacting with the above protein-based assemblies. Effect of partial charges with different spatial distribution will be considered in the atomistic molecular dynamics simulations. Atom trajectories will be recorded throughout the entire simulations for postprocessing the diffusion coefficient and analyzing the interactive behaviors.
April 2020