High Performance Li-S Batteries
Apparao Rao, R.A. Bowen Professor, Department of Physics and Astronomy, Clemson University - PI
A Stimulus Research Program Award Supported by the SC EPSCoR Program
(SC EPSCoR SRP Award 18-SR03)
The Research Team:
- Apparao Rao, R.A. Bowen Professor, Department of Physics and Astronomy, Clemson University - PI
- Ramakrishna Podila, Assistant Professor, Department of Physics and Astronomy, Clemson University – Co-PI
- Ming Hu, Associate Professor, Department of Mechanical Engineering, University of South Carolina, Co-PI
- John Weidner, Former Chair of Chemical Engineering, University of South Carolina, Co-PI
- Marlena Washington, Assistant Professor, Claflin University, Co-PI
- Narayanan Kuthirummal, Professor and Chair, Department of Physics and Astronomy, College of Charleston, Co-PI
The team is innovating battery technologies to transcend the limitation of lithium ion batteries through the development of “beyond-lithium ion” batteries such as the lithium sulfur, aluminum ion, sodium ion and potassium ion batteries. Key components of a battery include the anode that is electrically insulated from the cathode by a separator, and the electrolyte. In this work, novel cathodes, anodes and electrolytes were developed for high energy high power density batteries that are safe, and meet the needs of NASA space missions and the automotive industry.
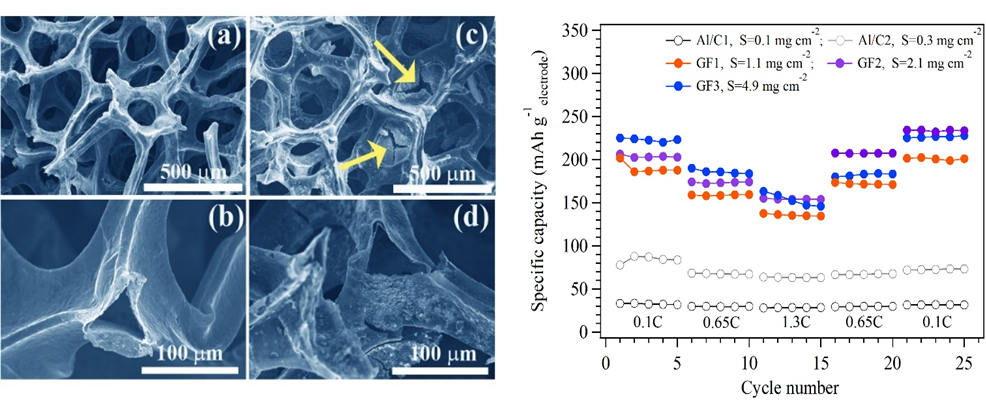
Porous carbon materials are ideally suited for battery electrodes as they are light weight, support faster ion diffusion, form robust electrode-electrolyte interfaces, and exhibit excellent interfacial charge transfer characteristics. We developed graphene foam based porous cathodes that contain high amounts of sulfur in the form of sulfurized polyacrylonitrile or SPAN (Figure 1A). A single sulfur atom can host a maximum of two electrons and sulfur has a theoretical specific capacity of 1675 mAh/g, which is eleven times higher than that of the widely used LiCoO2 (∼140 mAh/g) cathode. We demonstrated that the graphene foam cathodes outperform the conventional SPAN coated aluminum foils at the electrode level (Figure 1B). Thus, this study highlighted that a porous current collector, such as the graphene foam, facilitates high mass loading of SPAN without any deterioration in capacity with only one-third the weight of sulfur used in previous studies for achieving similar areal capacity.
 |
|
Figure 1: A) Scanning electron microscopy images of freestanding graphene-based cathodes. (a-b) pristine graphene foam; (c-d) graphene foam loaded with SPAN. The arrows in (c) show the presence of cracks in the infiltrated SPAN. Despite such cracks in SPAN, the graphene foam supports good electrical connectivity within SPAN. B) Specific capacity (normalized by the weight) of the electrodes at different C-rates; the cathodes used wither the graphene foam or aluminum current collectors. . |
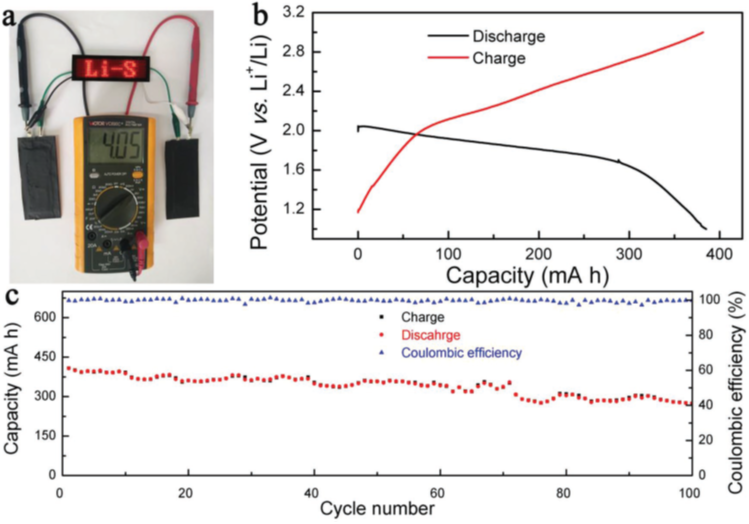
In addition to increasing the sulfur loading in the cathode, several other issues plague the rechargeable lithium sulfur batteries which impede their practical applications. During the first charge-discharge cycle of the battery the electrolyte forms a passive layer, called the “Solid-Electrolyte Interphase or SEI”, which comprises of inorganic and organic electrolyte decomposition products on both electrodes. The high resistances of sulfur and the lithium polysulfide intermediates, along with their structural changes, result in an unstable electrochemical contact within the sulfur cathode. Overall, the structural changes, increased internal resistance, unstable SEI layer, and dendrite growth result in a low utilization of sulfur, poor cycle life, and low system efficiency. Working together with Prof. Bingan Lu’s team (Hunan University, China), we developed a new electrolyte for lithium sulfur batteries to promote the simultaneous formation of bilateral SEIs on the sulfur-host cathode and the lithium anode, thus effectively suppressing the shuttle effect and dendrite growth. Importantly, we demonstrated long cycle stability of industrial grade high-capacity lithium sulfur pouch cells with new electrolytes, which could serve as a basis for robust energy dense lithium sulfur batteries (Figure 2).
 |
|
Figure 2: Industrial grade lithium sulfur pouch cells with capacity around 400 mA h. a) Photograph of the LED array which reads “Li-S.” The LED array was powered by two lithium sulfur pouch cells. b) Typical discharge–charge profiles. c) Cycling stability for 100 cycles. . |
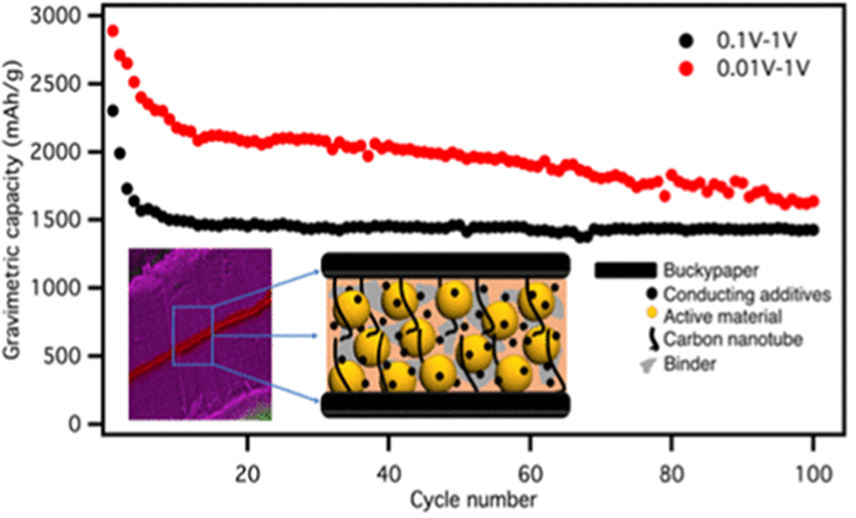
With respect to the anodes, Si has received much attention because of its high gravimetric (4200 mAh/g) and volumetric (9786 mAh/cm3) capacities. However, the practical application of Si anodes has been impeded by the low electrical conductivity of Si, large volume expansion upon lithiation (~400%) during first charge cycle, and pulverization during subsequent cycles. To prepare high performance anodes, we initially focused on the parent lithium ion batteries instead of the beyond lithium ion batteries. To this end, we prepared sandwich type anodes in which Si nanoparticles (average dia. ~30 nm and ~100 nm) were sandwiched between two freestanding carbon nanotube papers, or Buckypapers (Figure 3). Our detailed electrochemical impedance spectroscopy studies revealed that the sandwich electrodes exhibit a significantly reduced Warburg impedance and current collector active material interfacial resistance. Consequently, our sandwich anodes outperformed traditional anodes (comprised of Si nanoparticles on traditional copper foils) with a capacity as high as ∼500 mAh/g after 500 cycles at 0.1 C for 100 nm particles and up to ∼1490 mAh/g after 100 cycles at 0.1 C for 30 nm Si nanoparticles when discharged to 0.1 V. Furthermore, we found that the sandwich anodes are stable even at high C-rates - as high as 4 C.
The significance of the project and plans for future work: This study helped develop new strategies for the electrodes and electrolytes, which the team plans to leverage in the near future to develop other battery chemistries such as the sodium ion and potassium ion batteries. We will explore the possibility of using graphene foam as porous current collectors for sodium and potassium ion batteries. As the composition and stability of the SEI layer is dependent on both the type of electrolyte and the electrode material, we investigate the suitability of broadly using the carbonate based electrolytes because of their low cost compared to ether based electrolytes. The team is actively pursuing federal funding and partnership with industry to elevate battery research and workforce development in the State of South Carolina.
 |
|
Figure 3: Capacity of the sandwich type silicon anodes developed in this study. The silicon nanoparticles represent the active material which is mixed with a conducting additive and a binder and sandwiched between two Buckypapers. . |
